Protein-DNA interactions mediate many different epigenetic processes. Researchers studying these interactions need a reliable means of analyzing when and where they occur on the genome. ChIP is widely used to analyze protein-DNA interactions to specific genes and regulatory regions. Coupling ChIP with quantitative PCR (qPCR) provides a powerful tool to generate quantitative, real-time data on protein-DNA interactions within a short turnaround time.
A successful ChIP-qPCR experiment requires antibodies that are sensitive, specific, and provide reproducible results every time. CST rigorously validates the antibodies recommended for ChIP-qPCR to ensure they meet the high quality standards needed for reliable data generation.
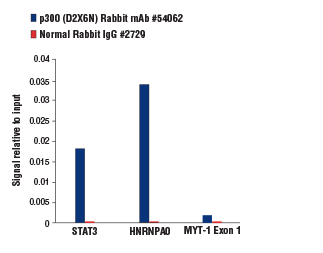
Chromatin immunoprecipitations were performed with cross-linked chromatin from K-562 cells and either p300 (D2X6N) Rabbit mAb #54062 or Normal Rabbit IgG #2729 using SimpleChIP® Plus Enzymatic Chromatin IP Kit (Magnetic Beads) #9005. The enriched DNA was quantified by real-time PCR using human STAT3 promoter primers, SimpleChIP® Human HNRNPA0 Promoter Primers #83602, and SimpleChIP® Human MYT-1 Exon 1 Primers #4493. The amount of immunoprecipitated DNA in each sample is represented as signal relative to the total amount of input chromatin, which is equivalent to one. The p300 (D2X6N) Rabbit mAb provided >8-fold enrichment of the STAT3 and HNRNPA0 positive loci as compared to the MYT-1 Exon 1 negative locus (compare the height of the blue bars for the STAT3 and HNRNPA0 positive loci to the MYT-1 Exon 1 negative locus).
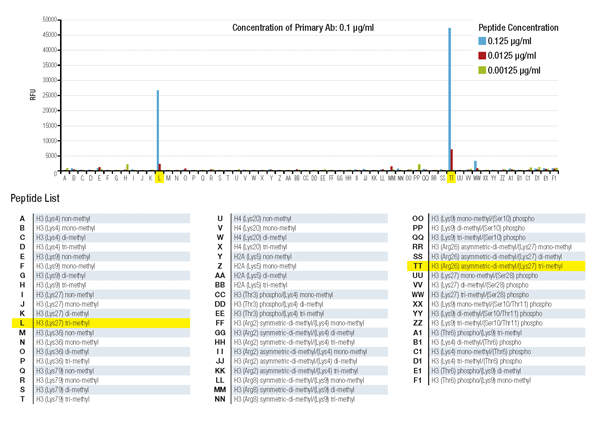
Histone peptide array assay demonstrates that Tri-Methyl-Histone H3 (Lys27) (C36B11) Rabbit mAb #9733 is highly specific for tri-methyl-histone H3 lysine 27 and is not affected by methylation of the neighboring arginine 26 residue.

Chromatin immunoprecipitations were performed with cross-linked chromatin from K-562 cells and either p300 (D2X6N) Rabbit mAb #54062 or Normal Rabbit IgG #2729 using SimpleChIP® Plus Enzymatic Chromatin IP Kit (Magnetic Beads) #9005. The enriched DNA was quantified by real-time PCR using human STAT3 promoter primers, SimpleChIP® Human HNRNPA0 Promoter Primers #83602, and SimpleChIP® Human MYT-1 Exon 1 Primers #4493. The amount of immunoprecipitated DNA in each sample is represented as signal relative to the total amount of input chromatin, which is equivalent to one. The p300 (D2X6N) Rabbit mAb provided >50-fold enrichment of the STAT3 and HNRNPA0 positive loci as compared to background enrichment of these same loci by the normal rabbit IgG (compare the height of the blue and the red bars for each target locus).
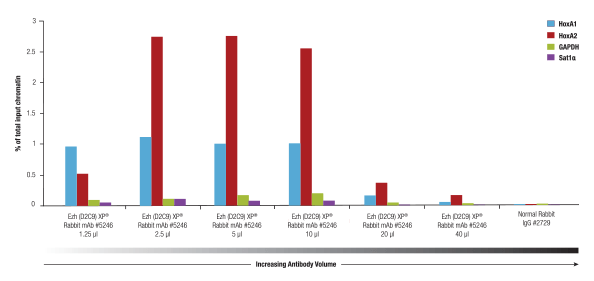
Ezh2 (D2C9) XP® Rabbit mAb #5246 was titrated using the SimpleChIP® Plus Enzymatic Chromatin IP Kit #9005 on cross-linked chromatin prepared from 4x10 NCCIT cells. This antibody showed optimal performance when used at 2.5 to 5 µl per IP.
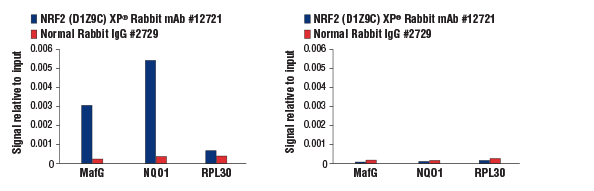
Chromatin immunoprecipitations were performed with cross-linked chromatin from MEF NRF2 wild-type (left) and NRF2 knock-out (right) cells, both treated with DEM (50 μM, 3 hr), and NRF2 (D1Z9C) XP® Rabbit mAb #12721 or Normal Rabbit IgG #2729. The enriched DNA was quantified by real-time PCR using mouse MafG intron 1 primers, SimpleChIP® Mouse NQO1 Promoter Primers #12635, and SimpleChIP® Mouse RPL30 Intron 2 Primers #7015. The amount of immunoprecipitated DNA in each sample is represented as signal relative to the total amount of input chromatin, which is equivalent to one. As expected, NRF2 (D1Z9C) XP® Rabbit mAb showed no enrichment of the MafG and NQ01 positive loci in the knock-out cells (right).
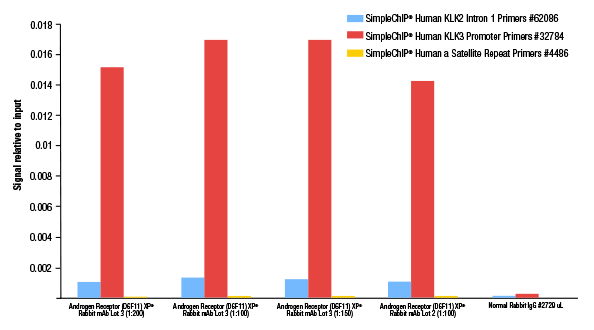
Chromatin immunoprecipitations were performed with cross-linked chromatin from LNCaP cells grown in phenol red free medium and 5% charcoal stripped FBS for 3 days then treated with dihydrotestosterone (DHT, 10 nM) for 4 hours, using SimpleChIP® Plus Enzymatic Chromatin IP Kit (Magnetic Beads) #9005. In this experiment, lot 3 of Androgen Receptor (D6F11) XP® Rabbit mAb #5153 was titrated and compared to the previously determined 1:100 optimal dilution of lot 2 of the antibody. The enriched DNA was quantified by real-time PCR using SimpleChIP® Human KLK2 Intron 1 Primers #62086, SimpleChIP® Human KLK3 Promoter Primers #32784, and SimpleChIP® Human α Satellite Repeat Primers #4486. The amount of immunoprecipitated DNA in each sample is represented as signal relative to the total amount of input chromatin, which is equivalent to one. As shown, both lot 2 and lot 3 of this antibody show optimal performance at a 1:100 dilution.